Cyanine dyes
Cyanine dyes are molecules containing polymethine bridge between two nitrogen atoms with a delocalized charge:
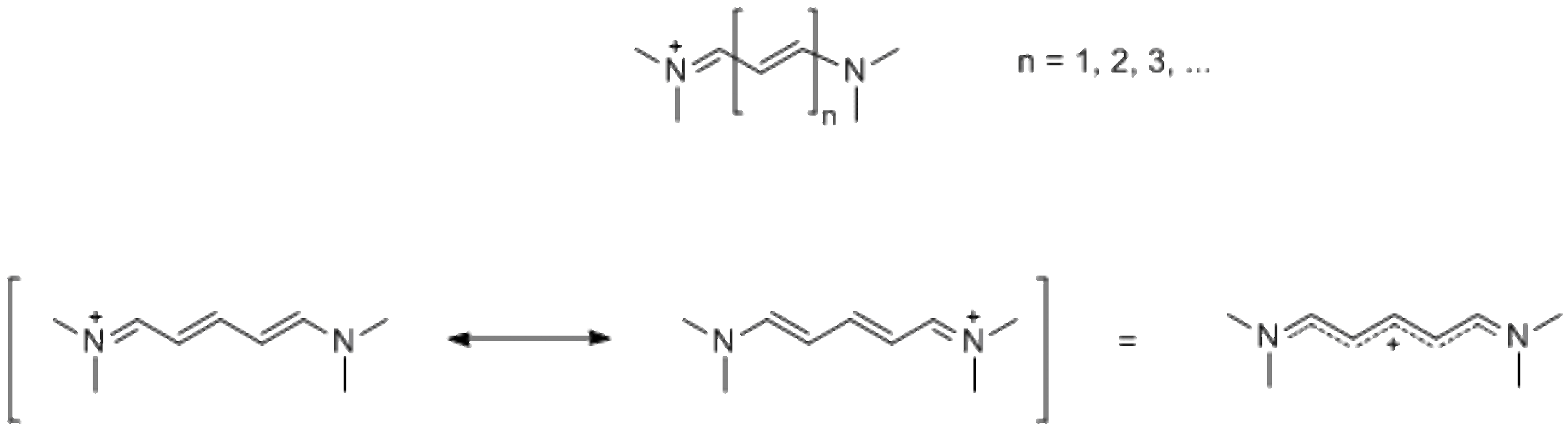
Due to their structure, cyanines have outstandingly high extinction coefficients often exceeding 100,000 Lmol-1cm-1. Different substituents allow to control properties of the chromophore, such as absorbance wavelength, photostability, and fluorescence. For example, absorbance and fluorescence wavelength can be controlled by a choice of polymethine bridge length: longer cyanines possess higher absorbance and emission wavelengths up to near infrared region.
A number of cyanine dyes have been used for life science applications. A series of thiazole and oxazole dyes have been used as DNA- and protein-binding dyes (like TOTO, YOYO, Stains All and others). But the most popular cyanine dyes for life science research were introduced by Alan Waggoner and colleagues of Carnegie Mellon University in early 1990s. The dyes were a modification of cyanine dye Indocyanine Green (ICG) which was used for angiography since 1970s, and they all contain two indolenine rings flanking polymethyne chain. The dyes were found to exhibit low non-specific binding to biomolecules, and have bright fluorescence owing to their huge extinction coefficients and good quantum yields. Once patented, these molecules are now in public domain after expiration of patents of CMU, and are available for purchase from Lumiprobe for research and commercial use as various reactive derivatives, such as NHS esters, maleimides, azides for Click chemistry, and other derivatives.
There are two varieties of cyanine dyes: non-sulfonated cyanines, and sulfonated cyanines. For many applications they are interchangeable, because their spectral properties are nearly identical. Both sulfonated and non-sulfonated dyes can be used for the labeling of biomolecules such as DNA and proteins. The difference between the dyes is their solubility: sulfo- dyes are water-soluble, and they do not use of organic co-solvent for the labeling in aqueous environment. They are less prone to aggregation in water. There are cases when one of the type of cyanines is desired (see Sulfonated vs non-sulfonated cyanines section below).
Non-sulfonated cyanines
Available non-sulfonated dyes incude Cy3, Cy3.5, Cy5, Cy5.5, Cy7, and Cy7.5. Cy® stands for 'cyanine', and the first digit identifies the number of carbon atoms between the indolenine groups. Cy2 which is an oxazole derivative rather than indolenin, is an exception from this rule. The suffix .5 is added for benzo-fused cyanines. Variation of the structures allows to change fluorescence properties of the molecules, and to cover most important part of visible and NIR spectrum with several fluorophores.
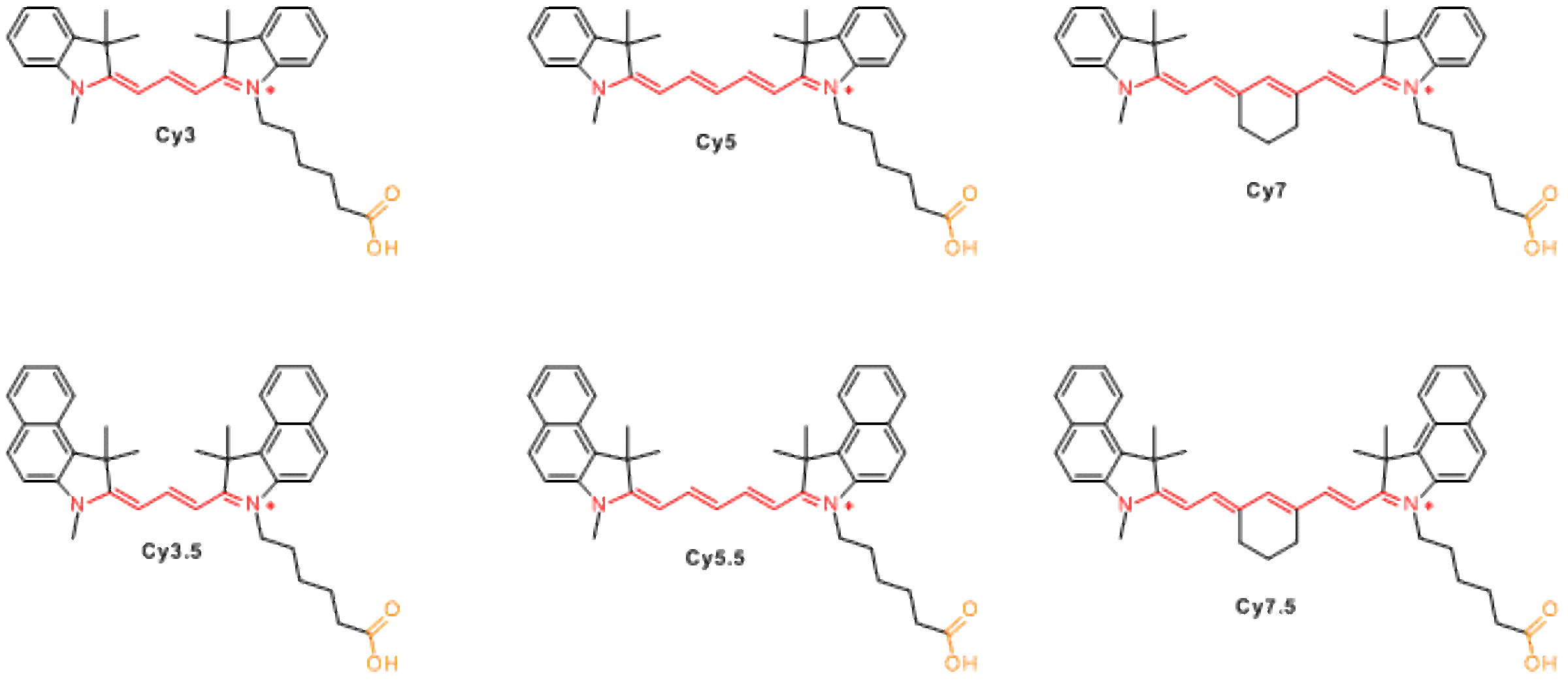
Most derivatives of non-sulfonated cyanines (except for hydrochlorides of hydrazides and amines) have low aqueous solubility. When these molecules are used for biomolecule labeling, use of organic co-solvent (5-20% of DMF or DMSO) is necessary for efficient reaction. Cyanine dye should be dissolved in organic solvent first, and added to a solution of biomolecule (protein, peptide, amino-labeled DNA) in appropriate aqueous buffer. When conjugation takes place efficiently, the dye reacts before it precipitates.
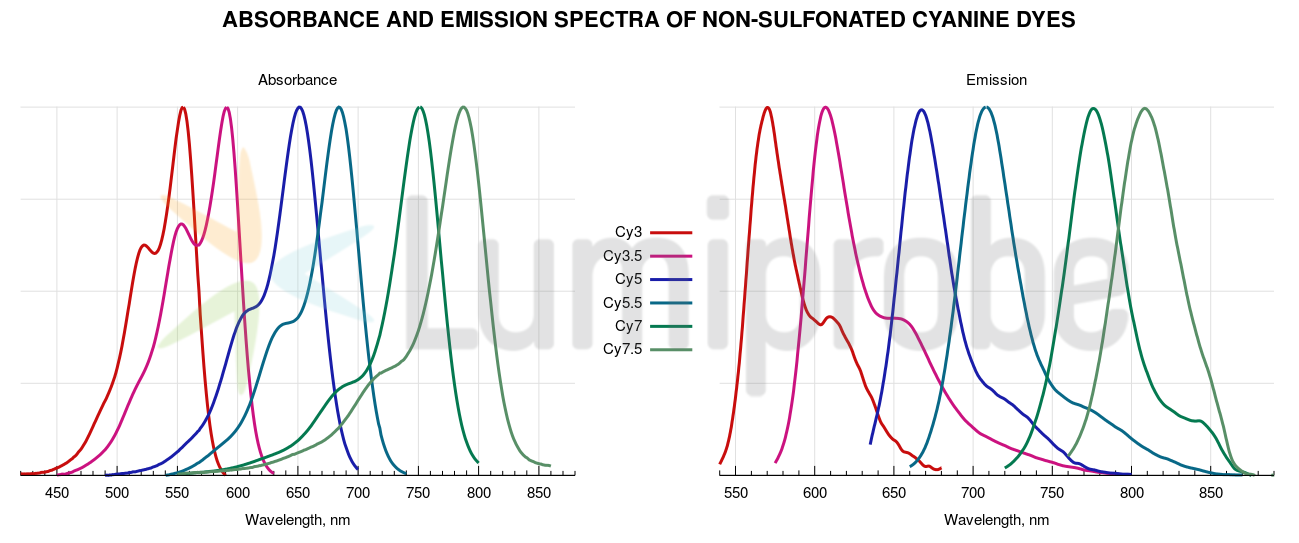
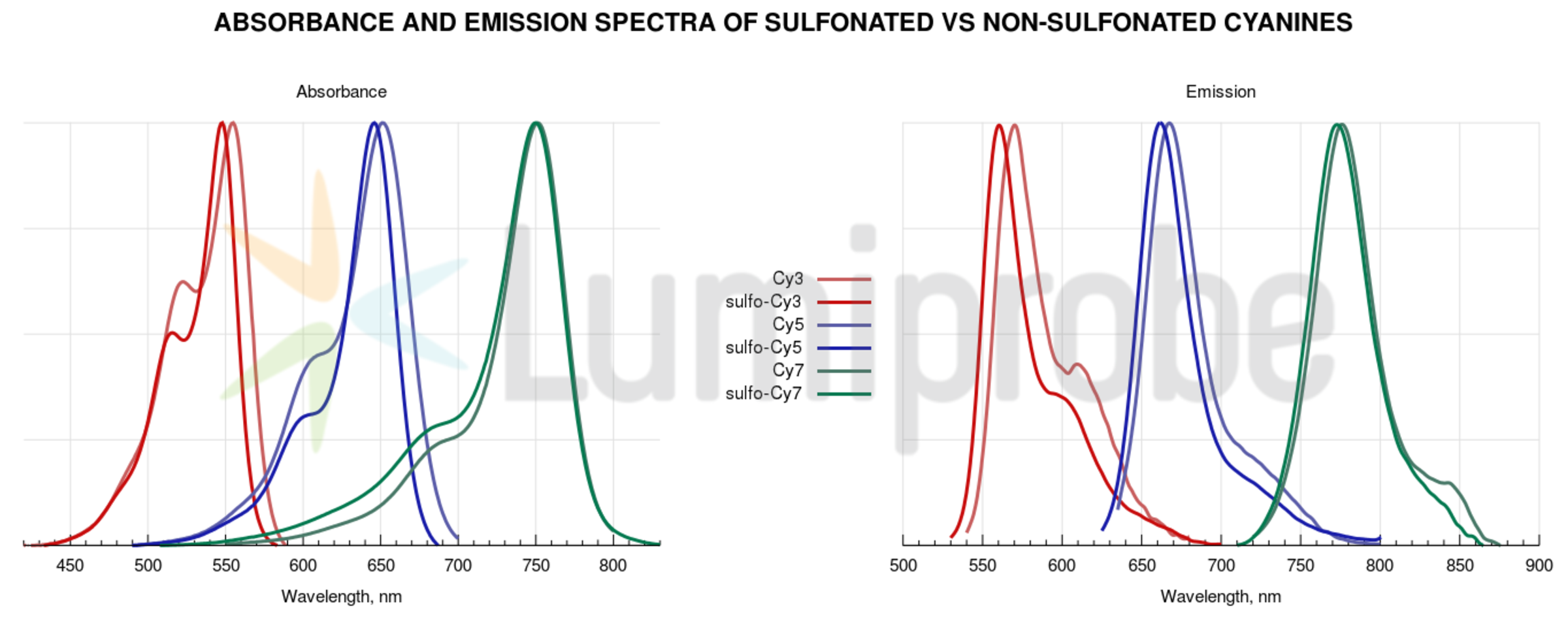
Fluorescent properties of non-sulfonated cyanines have little dependence on solvent and surrounding. Absorbance and fluorescence spectra of non-sulfonated cyanine dyes are plotted below.
Sulfonated cyanines
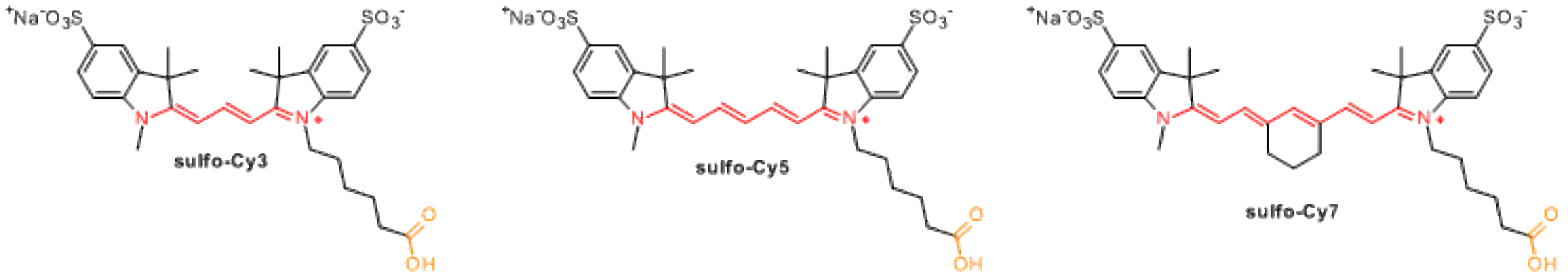
Sulfonated cyanines include additional sulfo-groups which facilitate dissolution of dye molecules in aqueous phase. Charged sulfonate groups decrease aggregation of dye molecules and heavily labeled conjugates.
Currently available sulfonated cyanines include sulfo-Cy3, sulfo-Cy5, and sulfo-Cy7.
Sulfonated cyanines are highly water soluble. No organic co-solvent is needed to perform labeling with these reagents.
Sulfonated vs non-sulfonated cyanines
Sulfonated and non-sulfonated cyanines exhibit very similar fluorescent properties. However, there are a few differences in labeling protocols that should be noticed. Non-sulfonated cyanines must be dissolved in an organic co-solvent (DMF or DMSO) prior to use, and added to a solution of the target molecule in aqueous buffers. The recommended volume of co-solvent should be 10% for Cy3, Cy5, Cy7, and 15% for .5 counterparts. Sulfo-Cyanine reagents can be used in purely aqueous conditions. There is also a difference in purification: when dialysis against water or aqueous buffer is used for purification, sulfo-Cyanine must be used to achieve efficient removal of unreacted dye material. Reactions with both sulfo- and non-sulfo cyanines can be purified by gel filtration, chromatography (HPLC, FPLC, ion exchange), or electrophoresis.
Sulfonated and non-sulfonated cyanines are interchangeable for the labeling of many classes of targets including:
- soluble proteins, which are tolerant to addition of organic co-solvent
- antibodies (use 5-10% of DMSO/DMF)
- DNA and oligonucleotides
- peptides
- many small molecules
Conjugates produced with similar sulfo- and non-sulfonated reagents (for example, sulfo-Cy5 and Cy5) are very similar in their fluorescent properties, and can be used with various fluorescence instrumentation.
Sulfonated cyanines must be used for:
- sensitive proteins which are denatured by DMF or DMSO
- protein conjugation when purification is done by dialysis
- nanoparticles in aqueous solutions
- insoluble or hydrophobic proteins
Non-sulfonated cyanines must be used for:
- reactions in organic media (dichloromethane, acetonitrile)
The following cyanine products are available from Lumiprobe.
| Fluorophore | Reactive form |
|---|---|
| sulfo-Cy3 | NHS ester maleimide azide alkyne carboxylic acid |
| sulfo-Cy5 | NHS ester maleimide azide alkyne carboxylic acid |
| sulfo-Cy7 | NHS ester azide amine carboxylic acid |
| Cy3 | NHS ester maleimide azide alkyne hydrazide amine carboxylic acid |
| Cy3.5 | NHS ester azide carboxylic acid |
| Cy5 | NHS ester maleimide azide alkyne hydrazide amine carboxylic acid |
| Cy5.5 | NHS ester maleimide azide alkyne hydrazide amine carboxylic acid |
| Cy7 | NHS ester maleimide azide alkyne hydrazide amine carboxylic acid |
| Cy7.5 | NHS ester maleimide azide alkyne hydrazide amine carboxylic acid |
Cy® is a trademark of GE Healthcare.






 $
$ 

