Cyanine5.5 alkyne
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| A70B0 | 1 mg |
$125
|
in stock | |
| B70B0 | 5 mg |
$260
|
in stock | |
| C70B0 | 10 mg |
$325
|
in stock | |
| D70B0 | 25 mg |
$510
|
in stock | |
| E70B0 | 50 mg |
$895
|
in stock | |
| F70B0 | 100 mg |
$1490
|
in stock |

Far red / near-infrared dye alkyne for сlick сhemistry labeling. Cyanine5.5 is an analog of Cy5.5®, a popular fluorophore widely used for various applications, including intact organism imaging. This reagent can be conjugated with azido groups under mild copper-catalyzed сlick сhemistry conditions.
This reagent is soluble in organic solvents, but mixtures of water with a small percent of DMSO can be used for efficient conjugation. Cyanine5.5 alkyne can also be used for the labeling of small molecules with this far red/NIR dye.
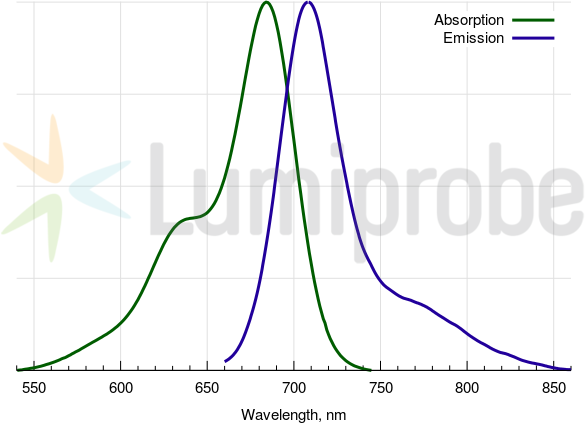
Absorbance and emission spectra of Cyanine5.5
Recommended protocol
Calculator
Customers also purchased with this product
General properties
| Appearance: | dark blue powder |
| Molecular weight: | 656.30 |
| CAS number: | 1628790-37-3 |
| Molecular formula: | C43H46ClN3O |
| Solubility: | good in organic solvents (DMF, DMSO, acetonitrile, DCM, alcohols), practically insoluble in water (5.2 uM, 3.7 mg/L) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 684 |
| ε, L⋅mol−1⋅cm−1: | 198000 |
| Emission maximum, nm: | 710 |
| Fluorescence quantum yield: | 0.2 |
| CF260: | 0.07 |
| CF280: | 0.03 |
Product citations
- Zhang, J.; Madge, H. Y. R.; Mahmoud, A.; Lu, L.; Wang, W.; Huang, W.; Koirala, P.; Gonzalez Cruz, J. L.; Kong, W. Y.; Bashiri, S.; Shalash, A. O.; Hussein, W. M.; Khalil, Z. G.; Wells, J. W.; Toth, I.; Stephenson, R. J. A Synthetic Cyclic Peptide for Promoting Antigen Presentation and Immune Activation. npj Vaccines, 2025, 10(1), 9. doi: 10.1038/s41541-024-01050-4
- Agnes, R.S.; Traughber, B.J.; Muzic, R.F. Development of a selective novel fluorescent substrate for sodium-dependent transporters. Life sciences, 2024, 351, 122847. doi: 10.1016/j.lfs.2024.122847
- Arranz-Gibert, P.; Vanderschuren, K.; Haimovich, A.; Halder, A.; Gupta, K.; Rinehart, J.; Isaacs, F. J. Chemoselective Restoration of Para-Azido-Phenylalanine at Multiple Sites in Proteins. Cell Chemical Biology, 2022, 29(6), 1046-1052.e4. doi: 10.1016/j.chembiol.2021.12.002
- Li, M.; Ruwe, H.; Melzer, M.; Junker, A.; Hensel, G.; Tschiersch, H.; Schwenkert, S.; Chamas, S.; Schmitz-Linneweber, C.; Börner, T.; Stein, N. The Arabidopsis AAC Proteins CIL and CIA2 Are Sub-functionalized Paralogs Involved in Chloroplast Development. Frontiers in Plant Science, 2021, 12, 681375. doi: 10.3389/fpls.2021.681375

























 $
$ 
