Quality control
ISO 9001:2015
The certificate No.:22.09.509-QM; Issued by Inspect;
Valid until 2025-11-01
Quality is a strong point of our company as your vendor. While we strive to provide affordable pricing - we never sacrifice quality for it. Here is a brief description of what we do to maintain quality.
Chemical analysis
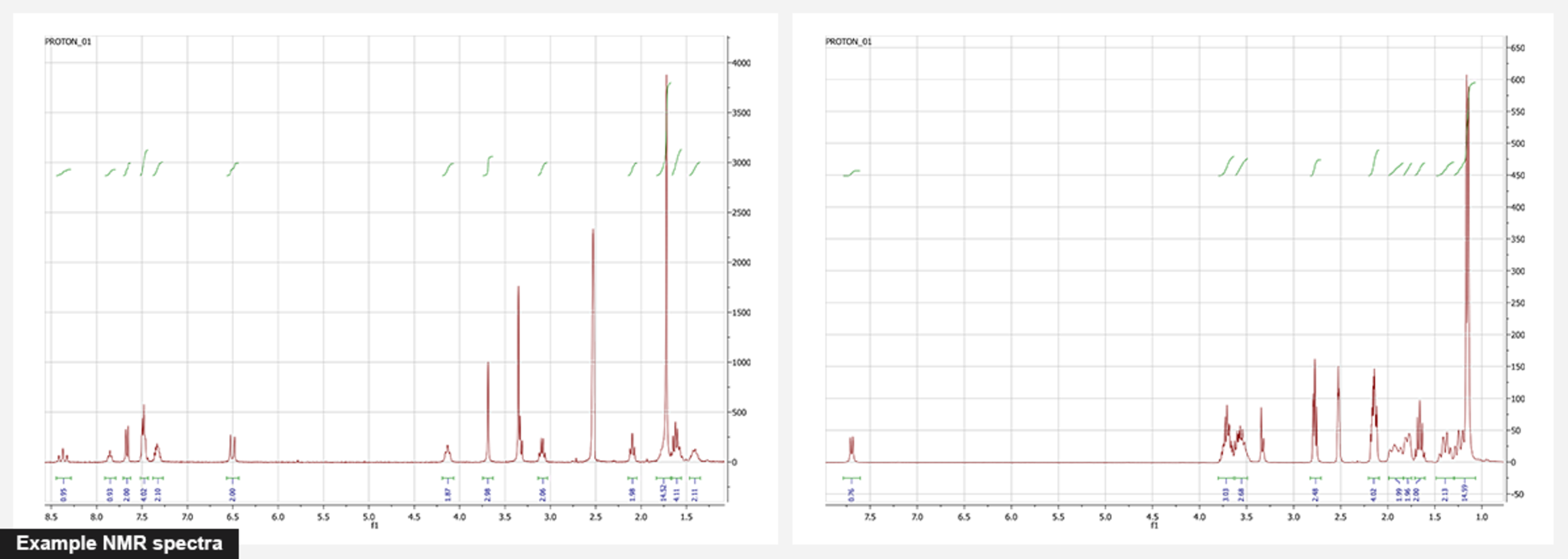
NMR. Nuclear magnetic resonance is the basic method for the identification of organic compounds. We routinely use 1H NMR for identity and purity checking. For phosphoramidites and triphosphates, 31P NMR is used, too. And we also measure other nuclei (13C, 19F, etc) for all compounds which we prepare for the first time to identify them unambiguously. Two-dimensional NMR (such as HSQC and HMBC) are used for complicated cases. For example, we attributed rhodamine isomers with 2D NMR - something impossible to do with one-dimensional NMR spectra.
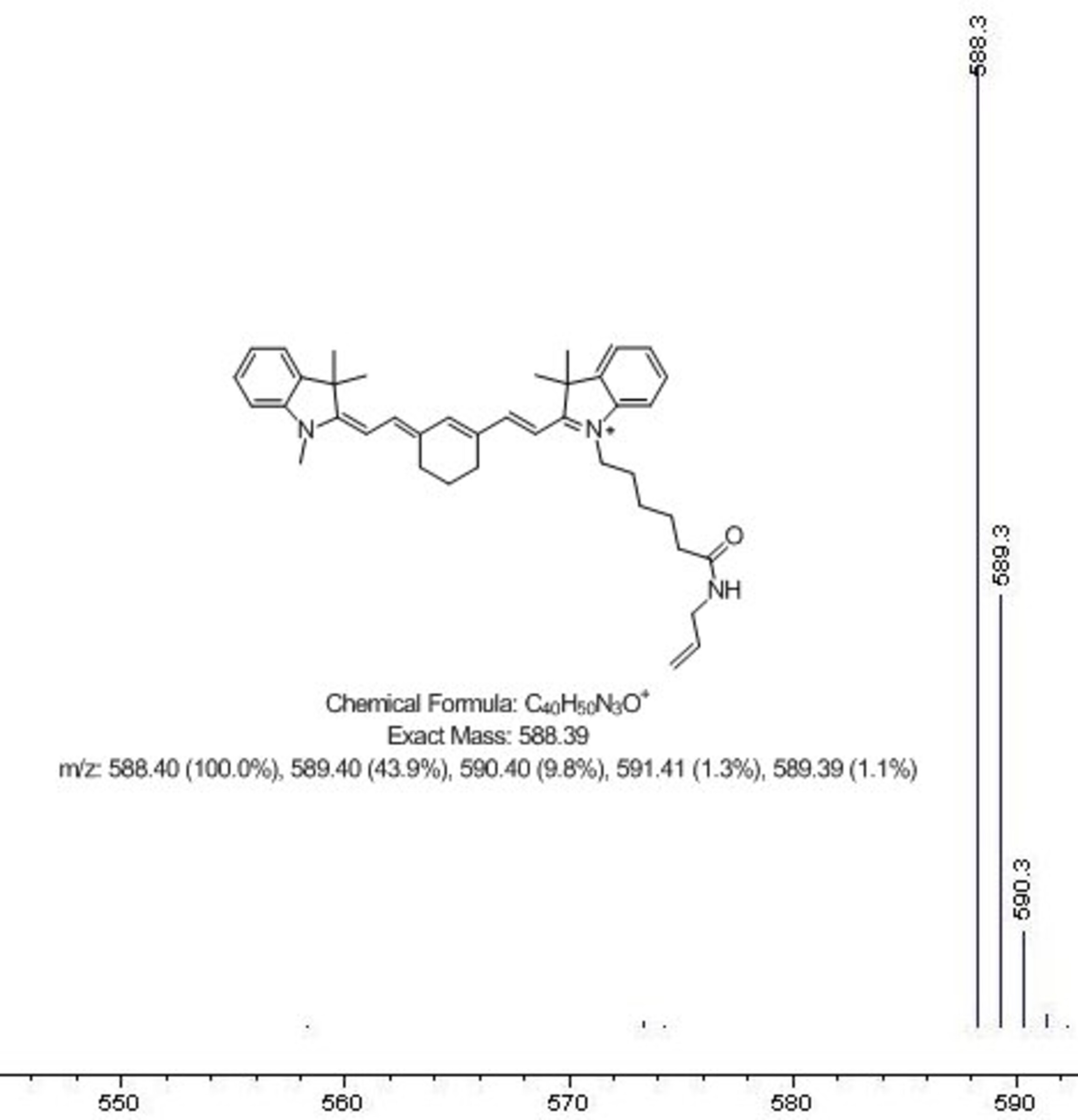
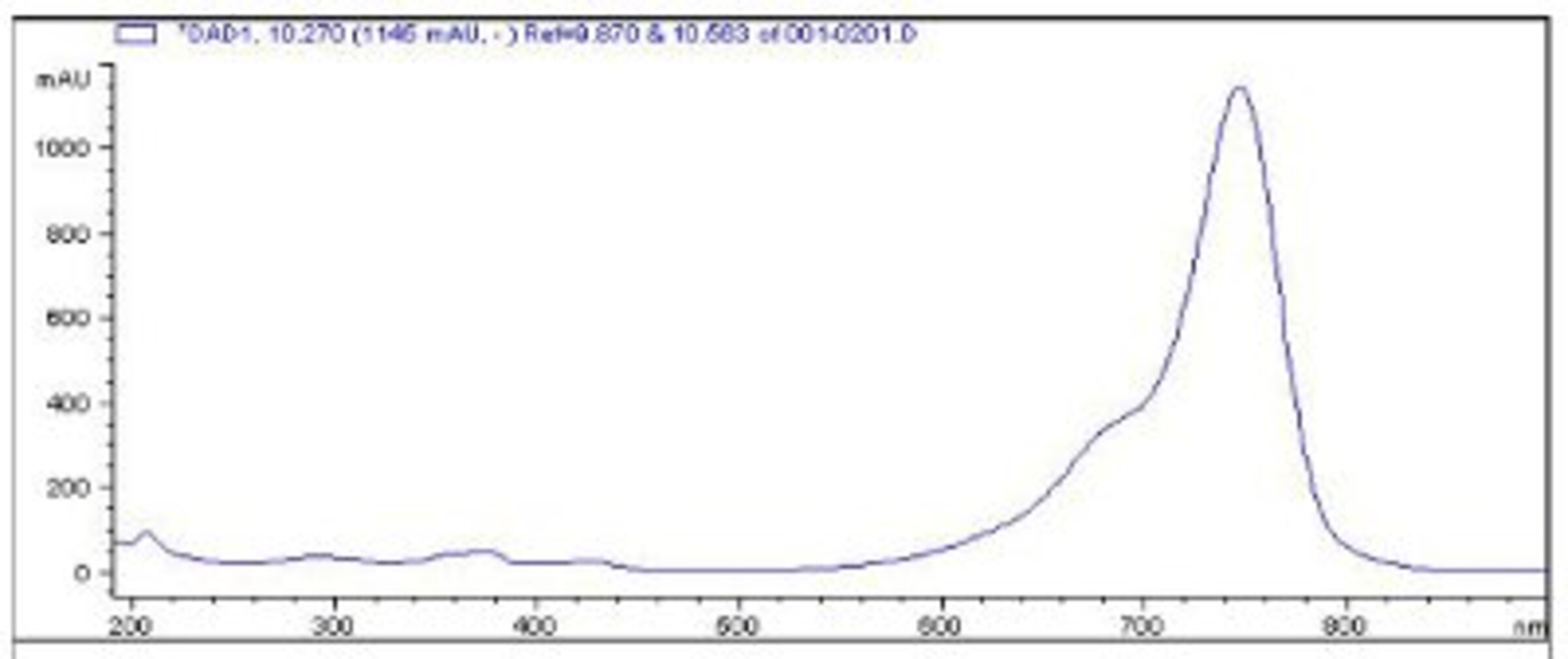
HPLC-MS. High pressure liquid chromatography coupled with mass spectrometry is a powerful method which we also routinely use for identity and purity checking. It gives quantitative measure of compound purity along with the measurement of its molecular weight.

This system that we have in house provides both UV spectra, and mass spectra for all of the components of the analyzed mixture - so we check online absorbance spectra of dyes, as well as their molecular weights.
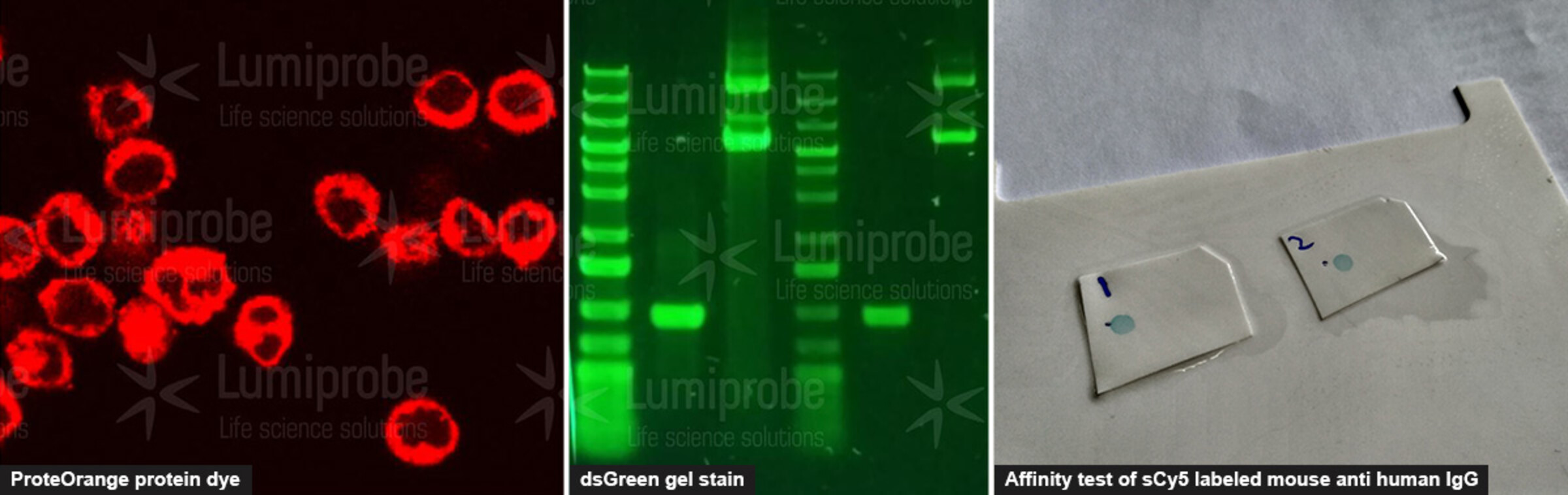
Functional testing. For our users, knowing that the compound works for the desired application is often the main thing. We regularly check that the reagents work as expected - for example, that they label proteins, stain gels, and provide fluorescent signals.
Batch numbers
Since end of 2012, we use batch numbering system which allows to identify each material that has been shipped to our customer.

We store sample of each material we obtained, and we can assay it again if something goes wrong at customer's lab. Batch number ensures that the material we have at our facility, and compound at customer's lab is the same.
Electronic quality system
Since August 2013, we maintain an electronic quality system which keeps records of all materials and intermediates produced and analyzed at our labs. All manufacturing steps are well documented, too. This allows to obtain consistent results and maintain outstanding quality over time.
Certificates of analysis
Quality of each batch is confirmed by certificate of analysis. The document is generated electronically by our quality system, and it contains most important analytical data for the particular batch of the material. You can download sample CoA here.







 $
$ 

