Cyanine5 amine
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 130C0 | 1 mg |
$125
|
in stock | |
| 230C0 | 5 mg |
$260
|
in stock | |
| 430C0 | 25 mg |
$510
|
in stock | |
| 530C0 | 50 mg |
$895
|
in stock | |
| 630C0 | 100 mg |
$1490
|
in stock |

Cyanine5 amine is a reactive dye which contains amino group, an analog of Cy5® amine. This reagent can be coupled with a variety of activated esters and other electrophilic reagents. For example, this amine can be coupled with EDC-activated carboxylic groups.
This bright and photostable dye is suitable for many different methods of fluorescence detection. Colorful fluorophore can also be easily detected in small quantities (nanomols) by naked human eye.
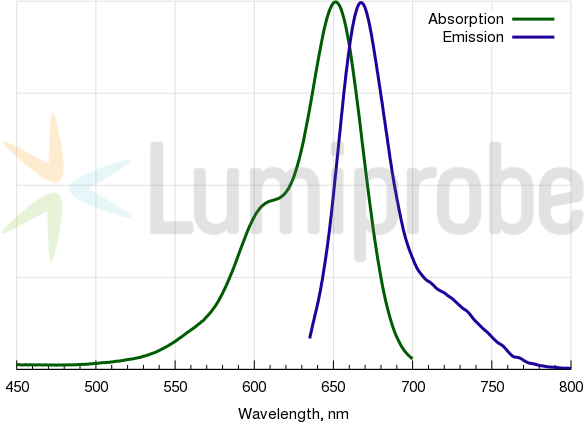
Cyanine5 amine absorbance and emission spectra
Customers also purchased with this product
General properties
| Appearance: | dark blue powder |
| Molecular weight: | 653.77 |
| CAS number: | 1807529-70-9 |
| Molecular formula: | C38H54Cl2N4O |
| IUPAC name: | 3H-Indolium, 2-[5-[1-[6-[(6-aminohexyl)amino]-6-oxohexyl]-1,3-dihydro-3,3-dimethyl-2H-indol-2-ylidene]-1,3-pentadien-1-yl]-1,3,3-trimethyl- |
| Solubility: | moderate solubility in water, good in polar organic solvents (DMF, DMSO, alcohols) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 646 |
| ε, L⋅mol−1⋅cm−1: | 250000 |
| Emission maximum, nm: | 662 |
| Fluorescence quantum yield: | 0.2 |
| CF260: | 0.03 |
| CF280: | 0.04 |
Product citations
- Gorshkova, M.; Vanchugova, L.; Osipova, N.; Nikitin, A.; Kotova, J.; Kovalenko, E.; Ermolenko, Y.; Malinovskaya, J.; Kovshova, T.; Gelperina, S. Hybrid DIVEMA/PLGA nanoparticles as the potential drug delivery system. Research Square, 2024, preprint. doi: 10.21203/rs.3.rs-4594368/v1
- Chau, Q.; Corado-Santiago, L.; Jones, S.; Dattelbaum, J.; Skromne, I. Physicochemical and Inflammatory Analysis of Unconjugated and Conjugated Bone-Binding Carbon Dots. ACS Omega, 2024, 9(1), 1320-1326. doi: 10.1021/acsomega.3c07653
- Yen, T.-Y.C.; Abbasi, A.Z.; He, C.; Lip, H.-Y.; Park, E.; Amini, M.A.; Adissu, H.A.; Foltz, W.; Rauth, A.M.; Henderson, J.; Wu, X.Y. Biocompatible and bioactivable terpolymer-lipid-MnO2 Nanoparticle-based MRI contrast agent for improving tumor detection and delineation. Materials Today Bio, 2024, 25, 100954. doi: 10.1016/j.mtbio.2024.100954
- Chen, J.; Wang, B.; Wang, Y.; Radermacher, H.; Qi, J.; Momoh, J.; Lammers, T.; Shi, Y.; Rix, A.; Kiessling, F. mRNA Sonotransfection of Tumors with Polymeric Microbubbles: Co‐Formulation versus Co‐Administration. Advanced Science, 2024, 11(15), 2306139. doi: 10.1002/advs.202306139
Cy® is a trademark of GE Healthcare.
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/1k
The count of items is incorrect.


























 $
$ 
