Cyanine3 amine
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 110C0 | 1 mg |
$125
|
in stock | |
| 210C0 | 5 mg |
$260
|
in stock | |
| 410C0 | 25 mg |
$510
|
in stock | |
| 510C0 | 50 mg |
$895
|
in stock | |
| 610C0 | 100 mg |
$1490
|
in stock |

Cyanine3 amine is a functionalized cyanine dye containing a free amino group. Cyanine3 is an analog of Cy3®.
Amino group of this reagent can be conjugated with reactive groups such as NHS esters, carboxy groups (after carbodiimide activation), and epoxides.
The amino dye is supplied in salt form, and possesses some aqueous solubility.
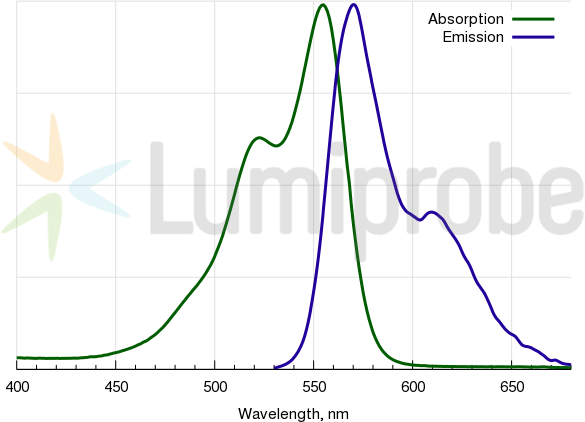
Cyanine3 absorption and emission spectra
Customers also purchased with this product
General properties
| Appearance: | red powder |
| Molecular weight: | 627.73 |
| CAS number: | 2247688-56-6 |
| Molecular formula: | C36H52Cl2N4O |
| IUPAC name: | 3H-Indolium, 1-[6-[(6-aminohexyl)amino]-6-oxohexyl]-2-[3-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)-1-propen-1-yl]-3,3-dimethyl-, chloride, hydrochloride (1:1:1) |
| Solubility: | moderate solubility in water, good in polar organic solvents (DMF, DMSO, alcohols) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 555 |
| ε, L⋅mol−1⋅cm−1: | 150000 |
| Emission maximum, nm: | 570 |
| Fluorescence quantum yield: | 0.31 |
| CF260: | 0.04 |
| CF280: | 0.09 |
Product citations
- Kovshova, T.; Malinovskaya, J.; Kotova, J.; Gorshkova, M.; Vanchugova, L.; Osipova, N.; Melnikov, P.; Vadekhina, V.; Nikitin, A.; Ermolenko, Y.; Gelperina, S. Core–Shell PLGA Nanoparticles: In Vitro Evaluation of System Integrity. Biomolecules, 2024, 14(12), 1601. doi: 10.3390/biom14121601
- Gorshkova, M.; Vanchugova, L.; Osipova, N.; Nikitin, A.; Kotova, J.; Kovalenko, E.; Ermolenko, Y.; Malinovskaya, J.; Kovshova, T.; Gelperina, S. Hybrid DIVEMA/PLGA nanoparticles as the potential drug delivery system. Research Square, 2024, preprint. doi: 10.21203/rs.3.rs-4594368/v1
- Lee, J.-R.; Sim, W.-S.; Park, H.-J.; Park, B.-W.; Joung, Y. K. Targeted Delivery of Apoptotic Cell-Derived Nanovesicles Prevents Cardiac Remodeling and Attenuates Cardiac Function Exacerbation. Advanced Functional Materials, 2023, 33(23), 2210864. doi: 10.1002/adfm.202210864
- Ruan, Z.; Li, S.; Grigoropoulos, A.; Amiri, H.; Hilburg, S. L.; Chen, H.; Jayapurna, I.; Jiang, T.; Gu, Z.; Alexander-Katz, A.; Bustamante, C.; Huang, H.; Xu, T. Population-Based Heteropolymer Design to Mimic Protein Mixtures. Nature, 2023, 615(7951), 251–258. doi: 10.1038/s41586-022-05675-0
Cy® is a trademark of GE Healthcare.
Short link - lumiprobe.com/sh/p/1U
The count of items is incorrect.


























 $
$ 
