Cyanine3 maleimide
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 11080 | 1 mg |
$125
|
in stock | |
| 21080 | 5 mg |
$260
|
in stock | |
| 41080 | 25 mg |
$510
|
in stock | |
| 51080 | 50 mg |
$895
|
in stock | |
| 61080 | 100 mg |
$1490
|
in stock |

Thiol mono-reactive Cyanine3 dye. This reagent can be used to attach Cyanine3 fluorophore (an analog of Cy3®) to proteins and peptides containing cysteine residues, as well as to other thiolated molecules (such as thiol-containing oligonucleotides).
Cystines should be reduced with TCEP (tris-carboxyethylphosphine) or other appropriate reductant prior to the labeling.
Labeling with Cyanine3 maleimide is selective, and efficient.
We recommend using water-soluble Sulfo-Cyanine3 maleimide for the labeling of antibodies and other sensitive proteins.
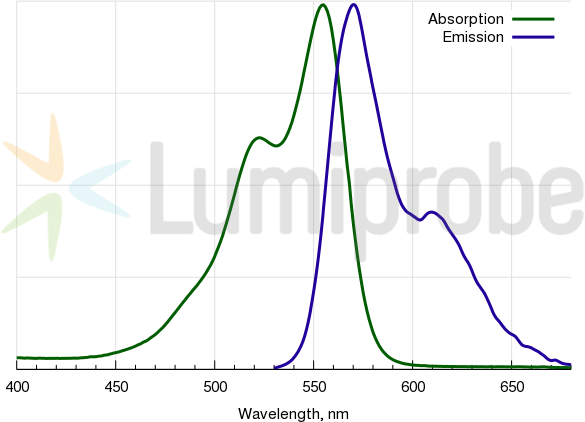
Cy3 absorbance and emission spectra
Recommended protocol
Calculator
Customers also purchased with this product
General properties
| Appearance: | red powder |
| Molecular weight: | 666.56 |
| Molecular formula: | C36H43N4O3BF4 |
| Solubility: | well soluble in DMSO (0.50 M = 330 g/L), in DMF, dichloromethane, very poorly soluble in water (0.57 mM = 420 mg/L) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 555 |
| ε, L⋅mol−1⋅cm−1: | 150000 |
| Emission maximum, nm: | 570 |
| Fluorescence quantum yield: | 0.31 |
| CF260: | 0.04 |
| CF280: | 0.09 |
Product citations
- Fischer, J.M.; Stewart, M.; Dai, M.; Drennan, S.; Holland, S.; Quentel, A.; Sabuncu, S.; Kingston, B.R.; Dengos, I.; Xiang, L.; Bonic, K.; Goncalves, F.; Yi, X.; Ranganathan, S.; Branchaud, B.P.; Muldoon, L.L.; Barajas, R.F.; Yildirim, A. Peptide Amphiphiles Hitchhike on Endogenous Biomolecules for Enhanced Cancer Imaging and Therapy. bioRxiv, 2024, preprint. doi: 10.1101/2024.02.21.580762
- Ernst, M.; Orabi, E.A.; Stockbridge, R.B.; Faraldo-Gómez, J.D.; Robertson, J.L. Dimerization mechanism of an inverted-topology ion channel in membranes. Proceedings of the National Academy of Sciences of the United States of America, 2024, 120(47), e2308454120. doi: 10.1073/pnas.2308454120
- Lin, R.; Wang, Y. Developing Multichannel SmFRET Approach to Dissecting Ribosomal Mechanisms. Chemical & Biomedical Imaging, 2024, 2(7), 501–509. doi: 10.1021/cbmi.4c00010
- Johnson, J.L.; Steele, J.H.; Lin, R.; Stepanov, V.G.; Gavriliuc, M.N.; Wang, Y. Multi-Channel smFRET study reveals a Compact conformation of EF-G on the Ribosome. bioRxiv, 2024, preprint. doi: 10.1101/2024.01.27.577133

























 $
$ 
