Cyanine5.5 carboxylic acid
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 17090 | 1 mg |
$125
|
in stock | |
| 27090 | 5 mg |
$260
|
in stock | |
| 47090 | 25 mg |
$510
|
in stock | |
| 57090 | 50 mg |
$895
|
in stock | |
| 67090 | 100 mg |
$1490
|
in stock |

Cyanine5.5 dye, free acid form, unactivated. The dye can be considered non-reactive for most applications. It can be used as a control or reference sample, and for instrument calibration.
Pre-activated NHS ester for the labeling of amine groups is also available.
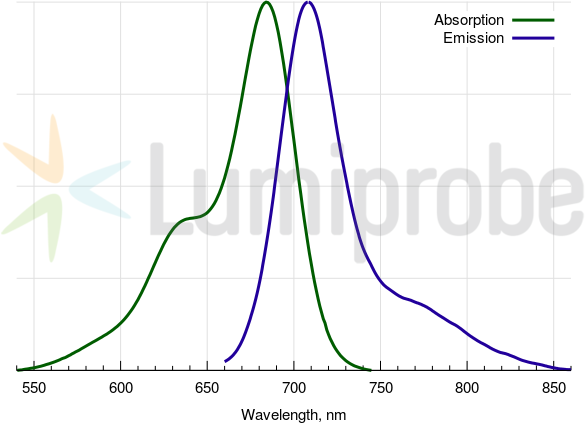
Cy5.5 absorbance and emission spectra
Customers also purchased with this product
General properties
| Appearance: | dark blue powder |
| Molecular weight: | 619.23 |
| CAS number: | 1449661-34-0 (without anion), 1449612-07-0 (inner salt) |
| Molecular formula: | C40H43ClN2O2 |
| IUPAC name: | 1H-Benz[e]indolium, 2-[5-[3-(5-carboxypentyl)-1,3-dihydro-1,1-dimethyl-2H-benz[e]indol-2-ylidene]-1,3-pentadien-1-yl]-1,1,3-trimethyl- |
| Solubility: | soluble in organic solvents (DMSO, DMF, dichloromethane), practically insoluble in water (< 1 uM, < 1 mg/L) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 684 |
| ε, L⋅mol−1⋅cm−1: | 198000 |
| Emission maximum, nm: | 710 |
| Fluorescence quantum yield: | 0.2 |
| CF260: | 0.07 |
| CF280: | 0.03 |
Product citations
- Shik, A. V.; Sobolev, P. V.; Zubritskaya, Y. V.; Baytler, M. O.; Stepanova, I. A.; Chernyaev, A. P.; Borschegovskaya, P. Yu.; Zolotov, S. A.; Doroshenko, I. A.; Podrugina, T. A.; Bliznyuk, U. A.; Rodin, I. A.; Beklemishev, M. K. Rapid Testing of Irradiation Dose in Beef and Potatoes by Reaction-Based Optical Sensing Technique. Journal of Food Composition and Analysis, 2024, 127, 105946. doi: 10.1016/j.jfca.2023.105946
- Anderson, C. F.; Wang, Q.; Stern, D.; Leonard, E. K.; Sun, B.; Fergie, K. J.; Choi, C.; Spangler, J. B.; Villano, J.; Pekosz, A.; Brayton, C. F.; Jia, H.; Cui, H. Supramolecular Filaments for Concurrent ACE2 Docking and Enzymatic Activity Silencing Enable Coronavirus Capture and Infection Prevention. Matter, 2023, 6(2), 583–604. doi: 10.1016/j.matt.2022.11.027
- Shik, A.V.; Stepanova, I.A.; Doroshenko, I.A.; Podrugina, T.A.; Beklemishev, M.K. Carbocyanine-Based Optical Sensor Array for the Discrimination of Proteins and Rennet Samples Using Hypochlorite Oxidation. Sensors (Basel), 2023, 23(9), 4299. doi: 10.3390/s23094299
- Lee, S.; Kim, D.; Kang, K.-K.; Sung, S.-E.; Choi, J.-H.; Sung, M.; Shin, C.-H.; Jeon, E.; Kim, D.; Kim, D.; Lee, S.; Kim, H.-K.; Kim, K. Toxicity and Biodistribution of Fragmented Polypropylene Microplastics in ICR Mice. International Journal of Molecular Sciences, 2023, 24(10), 8463. doi: 10.3390/ijms24108463
Cy® is a trademark of GE Healthcare.
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/Z
The count of items is incorrect.


























 $
$ 
