Cyanine5.5 amine
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 170C0 | 1 mg |
$125
|
5 days | |
| 270C0 | 5 mg |
$260
|
in stock | |
| 470C0 | 25 mg |
$510
|
in stock | |
| 570C0 | 50 mg |
$895
|
in stock | |
| 670C0 | 100 mg |
$1490
|
in stock |

Cyanine5.5 (Cy5.5® analog) amine derivative. The dye contains a free amine group which can be conjugated with a variety of functionalities, including NHS esters, and epoxides.
Cyanine5.5 is a far red dye which works fine for live organism imaging, and applications requiring low fluorescence background.
Cy5.5 absorbance and emission spectra

Customers also purchased with this product
General properties
| Appearance: | dark blue powder |
| Molecular weight: | 753.88 |
| CAS number: | 2097714-44-6 (without anion), 2097714-45-7 (chloride) |
| Molecular formula: | C46H58Cl2N4O |
| Solubility: | practically insoluble in water (< 2 uM), good in polar organic solvents (DMF, DMSO, alcohols) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 684 |
| ε, L⋅mol−1⋅cm−1: | 198000 |
| Emission maximum, nm: | 710 |
| Fluorescence quantum yield: | 0.2 |
| CF260: | 0.07 |
| CF280: | 0.03 |
Product citations
- Schvartzman, C.; Ibarboure, E.; Martin, A.; Garanger, E.; Mutschler, A.; Lecommandoux, S. Protocells Featuring Membrane-Bound and Dynamic Membraneless Organelles. Biomacromolecules, 2024, 25(7), 4087–4094. doi: 10.1021/acs.biomac.4c00200
- ten Hove, M.; Smyris, A.; Booijink, R.; Wachsmuth, L.; Hansen, U.; Alic, L.; Faber, C.; Hӧltke, C.; Bansal, R. Engineered SPIONs functionalized with endothelin a receptor antagonist ameliorate liver fibrosis by inhibiting hepatic stellate cell activation. Bioactive Materials, 2024, 39, 406-426. doi: 10.1016/j.bioactmat.2024.05.034
- Ayala-Orozco, C.; Li, G.; Li, B.; Vardanyan, V.; Kolomeisky, A. B.; Tour, J. M. How to Build Plasmon-Driven Molecular Jackhammers That Disassemble Cell Membranes and Cytoskeletons in Cancer. Advanced Materials, 2024, 36(14), 2309910. doi: 10.1002/adma.202309910
- Grosmanová, E.; Pola, R.; Filipová, M.; Henry, M.; Coll, J.‐L.; Etrych, T. Novel strategies for enhanced fluorescence visualization of glioblastoma tumors based on HPMA copolymers conjugated with tumor targeting and/or cell‐penetrating peptides. VIEW, 2024, 5(3), 20230116. doi: 10.1002/viw.20230116
Cy® is a trademark of GE Healthcare.
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/1J
The count of items is incorrect.
























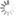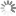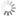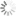
 $
$ 
