FAM maleimide, 6-isomer
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 24180 | 5 mg |
–
|
in stock | |
| 44180 | 25 mg |
$260
|
in stock | |
| 54180 | 50 mg |
$370
|
in stock | |
| 64180 | 100 mg |
$525
|
in stock |

FAM (fluorescein) is a bright fluorophore which is compatible with various fluorescence detection instruments. FAM is a universal dye that is useful for microscopy, qPCR and many other methods as well as for FRET-based and fluorescence polarization based binding assays.
FAM maleimide is a thiol reactive dye for the labeling of proteins, peptides, and other thiolated molecules.
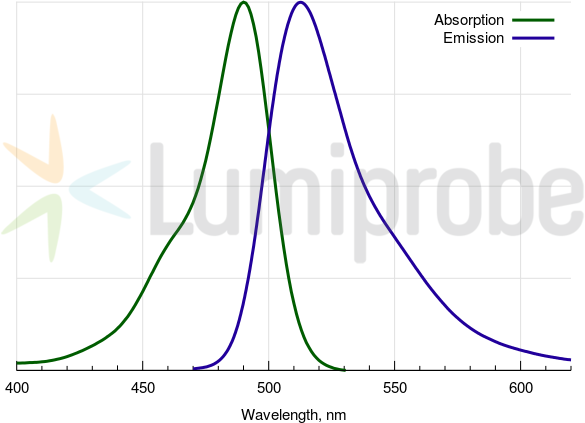
FAM absorbance and emission spectra
Recommended protocol
Calculator
Customers also purchased with this product
General properties
| Appearance: | yellow solid |
| Mass spec M+ increment: | 498.4 |
| Molecular weight: | 498.44 |
| Molecular formula: | C27H18N2O8 |
| Solubility: | good in DMSO, DMF |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 492 |
| ε, L⋅mol−1⋅cm−1: | 74000 |
| Emission maximum, nm: | 517 |
| Fluorescence quantum yield: | 0.93 |
| CF260: | 0.22 |
| CF280: | 0.17 |
Product citations
- Jang, K.; Kajale, S. N.; Joy, B. C.; Bono, D. C.; Neltner, B.; Sarkar, D. A Wearable Device for Continuous Monitoring of Circulating Cells at Single-Cell Resolution. npj Biosensing, 2025, 2(1), 10. doi: 10.1038/s44328-025-00032-3
- Huang, S.; Gao, Y.; Ma, L.; Jia, B.; Zhao, W.; Yao, Y.; Li, W.; Lin, T.; Wang, R.; Song, J.; Zhang, W. Design of pH-responsive antimicrobial peptide melittin analog-camptothecin conjugates for tumor therapy. Asian Journal of Pharmaceutical Sciences, 2024, 19(1), 100890. doi: 10.1016/j.ajps.2024.100890
- Gabashvili, A.N.; Alexandrushkina, N.A.; Mochalova, E.N.; Goliusova, D.V.; Sapozhnikova, E.N.; Makarevich, P.I.; Nikitin, P.I. Internalization of transferrin-tagged Myxococcus xanthus encapsulins into mesenchymal stem cells. Experimental Biology and Medicine, 2024, 249(3), 10055. doi: 10.3389/ebm.2024.10055
- Peterson, B. G.; Hwang, J.; Russ, J. E.; Schroeder, J. W.; Freddolino, P. L.; Baldridge, R. D. Deep Mutational Scanning Highlights a Role for Cytosolic Regions in Hrd1 Function. Cell Reports, 2023, 42(11), 113451. doi: 10.1016/j.celrep.2023.113451
Short link - lumiprobe.com/sh/p/2R
The count of items is incorrect.

























 $
$ 
