BDP® FL DBCO
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| A14F0 | 1 mg |
$125
|
in stock | |
| B14F0 | 5 mg |
$260
|
in stock | |
| C14F0 | 10 mg |
$325
|
in stock | |
| D14F0 | 25 mg |
$510
|
in stock | |
| E14F0 | 50 mg |
$895
|
in stock | |
| F14F0 | 100 mg |
$1490
|
in stock |

BDP FL is a photostable borondipyrromethene for the FAM (fluorescein) channel. The dye is compatible with filter sets for fluorescein. This reagent is a DBCO (azadibenzocyclooctyne) derivative for copper-free click chemistry reaction with azides.
Absorption and emission spectra of BDP FL

Customers also purchased with this product
General properties
| Appearance: | orange solid |
| Mass spec M+ increment: | 592.3 |
| Molecular weight: | 592.49 |
| CAS number: | 2360493-46-3 |
| Molecular formula: | C35H35N4BF2O2 |
| Solubility: | good in DMF, DMSO, DCM |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 503 |
| ε, L⋅mol−1⋅cm−1: | 92000 |
| Emission maximum, nm: | 509 |
| Fluorescence quantum yield: | 0.97 |
Product citations
- Merlo, R.; Caprioglio, D.; Cillo, M.; Valenti, A.; Mattossovich, R.; Morrone, C.; Massarotti, A.; Rossi, F.; Miggiano, R.; Leonardi, A.; Minassi, A.; Perugino, G. The SNAP-tag technology revised: an effective chemo-enzymatic approach by using a universal azide-based substrate. Journal of Enzyme Inhibition and Medicinal Chemistry, 2021, 36(1), 85–97. doi: 10.1080/14756366.2020.1841182
- Islam, M.R.; Nguy, C.; Pandit, S.; Lyon, L.A. Design and Synthesis of Core–Shell Microgels with One‐Step Clickable Crosslinked Cores and Ultralow Crosslinked Shells. Macromolecular Chemistry and Physics, 2020, 221(19), 2000156. doi: 10.1002/macp.202000156
BDP® ist eine Marke von Lumiprobe
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/4v
The count of items is incorrect.
























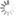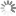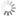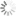
 $
$ 
