1-Ethynyl pyrene
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 515B0 | 50 mg |
$50
|
in stock | |
| 615B0 | 100 mg |
$64
|
in stock |

Pyrene is one of the simplest polyaromatic hydrocarbons (PAHs). Pyrene derivatives are known for their ability to intercalate dsDNA.
Pyrene possesses intrinsic fluorescence. When two pyrene residues are located in close proximity, excimer formation can be observed by fluorescence spectroscopy. Pyrene has been therefore used to probe structures of biomolecules.
Ethynylpyrene molecule contains terminal triple bond fragment for click chemistry, as well as other coupling reactions such as Sonogashira coupling.
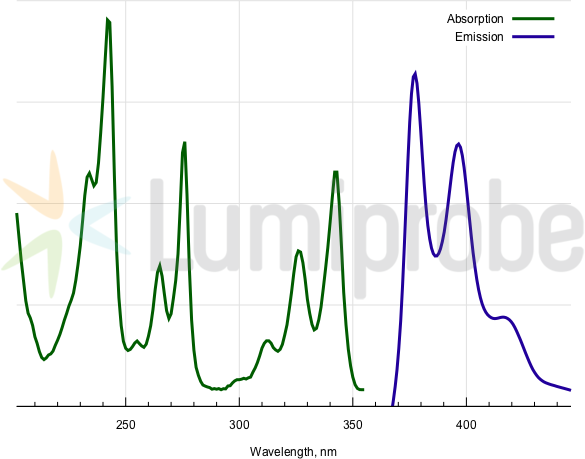
Absorption and emission spectra of pyrene fluorophore
Recommended protocol
Calculator
Customers also purchased with this product
General properties
| Appearance: | light yellow solid |
| Molecular weight: | 226.27 |
| CAS number: | 34993-56-1 |
| Molecular formula: | C18H10 |
| Solubility: | good in chloroform, dichloromethane, toluene, low in water |
| Quality control: | NMR 1H (95%) and 13C, TLC |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 343; 326; 313; 276; 265; 242; 234 |
| Emission maximum, nm: | 377; 397 |
Product citations
- Vega, B.; Wondraczek, H.; Bretschneider, L.; Näreoja, T.; Fardim, P.; Heinze, T. Preparation of reactive fibre interfaces using multifunctional cellulose derivatives. Carbohydrate Polymers, 2015, 132, 261–273. doi: 10.1016/j.carbpol.2015.05.048
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/1s
The count of items is incorrect.

























 $
$ 
