FAM phosphoramidite, 6-isomer
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
|
D5160
/ Easily soluble lyophilised form in 8 mL septum
|
250 mg |
$105
|
in stock | |
|
F5160
/ Easily soluble lyophilised form in 30 mL septum
|
1 g |
$167
|
in stock | |
| G5160 | 5 g |
$740
|
in stock | |
| H5160 | 10 g |
$970
|
in stock | |
| K5160 | 50 g |
please inquire
|
in stock | |
| L5160 | 100 g |
please inquire
|
in stock |

FAM (fluorescein) phosphoramidite 6-isomer is specifically designed for incorporation at the 5′-end of oligonucleotides, which is essential for many biochemical assays and applications. It is primarily utilized for labeling oligonucleotides, enhancing their visibility in various assays such as PCR, sequencing and hybridization studies. It is commonly employed in real-time PCR assays where fluorescent signals are monitored during amplification. The incorporation of FAM at the 5′-end allows for efficient monitoring of the reaction progress through fluorescence.
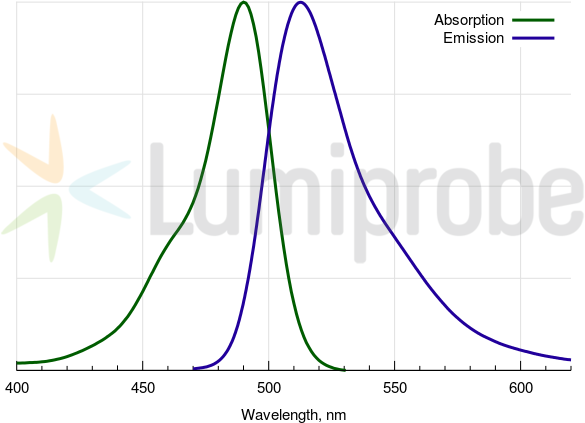
FAM emits fluorescence with a maximal emission wavelength around 517 nm, which is ideal for various detection methods. It can be quenched by agents like DusQ 1 or DABCYL, allowing for applications in assays that require precise control over fluorescence signals.
Internal sequence additions can be achieved using modified nucleotides such as FAM-dT phosphoramidite, allowing for flexible design of probes with multiple fluorescent tags while maintaining effective spacing. However, it is essential to include spacers between the labels to prevent self-quenching of fluorescence signals, which can occur due to close proximity.
The dye soluble in common organic solvents such as acetonitrile, DCM, DMF, DMSO. Once conjugated to biomolecules, the resulting FAM conjugates can be used in aqueous applications.
Product in action
FAM absorbance and emission spectra
Customers also purchased with this product
General properties
| Appearance: | off-white solid |
| Molecular weight: | 843.94 |
| CAS number: | 204697-37-0 |
| Molecular formula: | C46H58N3O10P |
| Solubility: | acetonitrile, DCM, DMF, DMSO |
| Quality control: | NMR 1H and 31P, HPLC-MS (95+%), isomeric purity > 97% |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 492 |
| ε, L⋅mol−1⋅cm−1: | 74000 |
| Emission maximum, nm: | 517 |
| Fluorescence quantum yield: | 0.93 |
| CF260: | 0.22 |
| CF280: | 0.17 |
Oligo synthesis details
| Diluent: | acetonitrile |
| Coupling conditions: | coupling time 10 min |
| Cleavage conditions: | ammonia, 2 h at room temperature |
| Deprotection conditions: | identical to protected nucleobases; when AMA is used, deblock with ammonia alone for 30 min, then add methylamine |
Product citations
- Zharkov, T.D.; Mironova, E.M.; Markov, O.V.; Zhukov, S.A.; Khodyreva, S.N.; Kupryushkin, M.S. Fork- and Comb-like Lipophilic Structures: Different Chemical Approaches to the Synthesis of Oligonucleotides with Multiple Dodecyl Residues. International Journal of Molecular Sciences, 2023, 24(19), 14637. doi: 10.3390/ijms241914637
- Bulgakova, A.; Chubarov, A.; Dmitrienko, E. Magnetic Nylon 6 Nanocomposites for the Microextraction of Nucleic Acids from Biological Samples. Magnetochemistry, 2022, 8(8), 85. doi: 10.3390/magnetochemistry8080085
- Stanton, J.-A.L.; O'Brien, R.; Hall, R.J.; Chernyavtseva, A.; Ha, H.J.; Jelley, L.; Mace, P.D.; Klenov, A.; Treece, J.M.; Fraser, J.D.; Clow, F.; Clarke, L.; Su, Y.; Kurup, H.M.; Filichev, V.V.; Rolleston, W.; Law, L.; Rendle, P.M.; Harris, L.D.; Wood, J.M.; Scully, T.W.; Ussher, J.E.; Grant, J.; Hore, T.A.; Moser, T.V.; Harfoot, R.; Lawley, B.; Quiñones-Mateu, M.E.; Collins, P.; Blaikie, R. Uncoupling Molecular Testing for SARS-CoV-2 From International Supply Chains. Frontiers in Public Health, 2022, 9, 808751. doi: 10.3389/fpubh.2021.808751
- Zhou, Z.; Liu, S.; Zhang, Y.; Yang, X.; Ma, Y.; Guan, Z.; Wu, Y.; Zhang, L.; Yang, Z. Reductive nanocomplex encapsulation of cRGD-siRNA conjugates for enhanced targeting to cancer cells. International Journal of Nanomedicine, 2017, 12, 7255–7272. doi: 10.2147/ijn.S136726


























 $
$ 
