dsGreen® Nucleic Acid Gel Staining Solution, 10,000×
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| A0010 | 50 uL |
–
|
in stock | |
| 10010 | 0.5 mL |
$135
|
in stock | |
| 20010 | 1 mL |
$229
|
in stock |

dsGreen® is a sensitive dsDNA binding dye, which can be used for routine DNA detection in agarose and polyacrylamide gels. A qPCR grade reagent is also available.
Unlike ethidium bromide, dsGreen is highly selective towards double stranded DNA, much less harmful, and offers better sensitivity.
Comparison between ethidium bromide and dsGreen
| Feature | Ethidium Bromide | dsGreen |
|---|---|---|
| Fluorescence | Red (615 nm) | Green (524 nm) |
| Excitation maximum | 302 nm | 454 nm |
| Excitation light source | UV only | Blue light or UV |
| Sensitivity | 2 ng / band (dsDNA) 100 ng / band (RNA) |
0.08 ng / band (dsDNA) 1–2 ng / band (oligonucleotides) |
| Health hazard | High | Low |
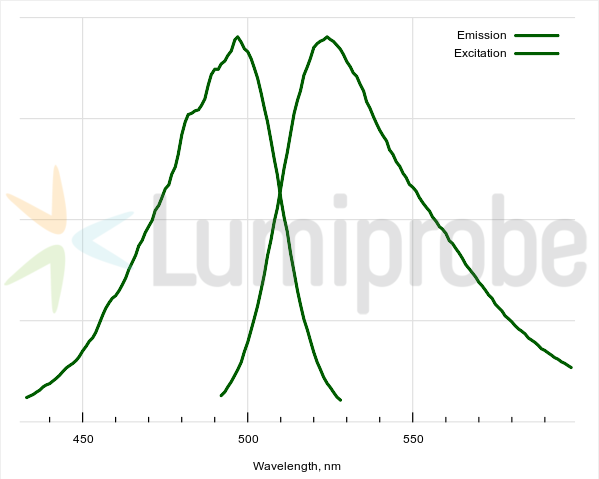
Excitation and emission spectra of dsDNA complex with dsGreen
Recommended protocol
Calculator
Customers also purchased with this product
General properties
| Appearance: | orange solution |
| Quality control: | UV-Vis abs |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 490 |
| ε, L⋅mol−1⋅cm−1: | 73000 |
| Emission maximum, nm: | 524 |
| Fluorescence quantum yield: | 0.8 |
Product citations
- Chetverikov, P. E.; Craemer, C.; Gankevich, V. D.; Kremenetskaya, M. V.; Kuzmin, I. V.; Zhuk, A. S. Endoparasitic Gall Mites: Two New Novophytoptus Species (Eriophyoidea, Phytoptidae) from Southern African Sedges (Cyperaceae, Carex) and New Hypotheses on the Phylogeny of Novophytoptines. Diversity, 2023, 15(3), 416;. doi: 10.3390/d15030416
- Gmoshinskiy, V. I.; Novozhilov, Yu. K.; Prikhodko, I. S.; Bortnikov, F. M.; Shchepin, O. N.; Schnittler, M. Morphology and phylogeny of Diderma aurantiacum (Myxomycetes) — a new species for Russia from the Far East. Novosti sistematiki nizshikh rastenii, 2023, 57(1), 27–42. doi: 10.31111/nsnr/2023.57.1.27
- Chetverikov, Ph.E.; Desnitskiy, A.G.; Klimov, P.B.; Ozman- Sullivan, S.K.; Romanovich, A.E.; Sukhareva, S.I. Deuterogyny and the Association of Two Vagrant Eriophyoid Mites (Acariformes, Eriophyoidea) with the Host-Plant Generative Organs of Two Broad-Leaved Trees in North-West Russia. Zoological Studies, 2023, 62, 35. doi: 10.6620/ZS.2023.62-35
- Abil, Z.; Restrepo Sierra, A. M.; Danelon, C. Clonal Amplification-Enhanced Gene Expression in Synthetic Vesicles. ACS Synth Biol, 2023, 12(4), 1187–1203. doi: 10.1021/acssynbio.2c00668
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/7
The count of items is incorrect.



























 $
$ 
