Cyanine3 NHS ester
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 11020 | 1 mg |
$91
|
in stock | |
| 21020 | 5 mg |
$119
|
in stock | |
| 41020 | 25 mg |
$380
|
in stock | |
| 51020 | 50 mg |
$670
|
in stock | |
| 61020 | 100 mg |
$1150
|
in stock | |
| 81020 | 1 g |
please inquire
|
in stock |

Cyanine3 NHS ester is a reactive dye for the labeling of amino-groups in biomolecules, an analog of Cy3® NHS ester. This reagent is ideal for the labeling of soluble proteins, peptides, and oligonucleotides/DNA. For delicate proteins consider using water-soluble sulfo-Cyanine3 NHS ester which does not require use of any co-solvent.
Cyanine3 NHS ester is a replacement for NHS esters of Cy3® and DyLight 549.
Absorption and emission spectra of Cyanine3

Customers also purchased with this product
General properties
| Appearance: | red powder |
| Mass spec M+ increment: | 439.5 |
| Molecular weight: | 641.5 |
| CAS number: | 2632339-91-2 |
| Molecular formula: | C34H40N3BF4O4 |
| IUPAC name: | 3H-Indolium, 2-[3-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)-1-propen-1-yl]-1-[6-[(2,5-dioxo-1-pyrrolidinyl)oxy]-6-oxohexyl]-3,3-dimethyl-, tetrafluoroborate |
| Solubility: | poorly soluble in water (2.3 mM = 1.5 g/L), soluble in organic solvents (DMF, DMSO, dichloromethane) |
| Quality control: | NMR 1H and HPLC-MS (95+%) |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 555 |
| ε, L⋅mol−1⋅cm−1: | 150000 |
| Emission maximum, nm: | 570 |
| Fluorescence quantum yield: | 0.31 |
| CF260: | 0.04 |
| CF280: | 0.09 |
Product citations
- Oh, H. J.; Lee, Y.; Hwang, H.; Hong, K.; Choi, H.; Kang, J. Y.; Jung, Y. Size-Controlled Assembly of Phase Separated Protein Condensates with Interfacial Protein Cages. Nat Commun, 2025, 16(1), 1009. doi: 10.1038/s41467-025-56391-y
- Deng, L.; Olea, A.R.; Ortiz‐Perez, A.; Sun, B.; Wang, J.; Pujals, S.; Palmans, A.R.A.; Albertazzi, L. Imaging Diffusion and Stability of Single‐Chain Polymeric Nanoparticles in a Multi‐Gel Tumor‐on‐a‐Chip Microfluidic Device. Small methods, 2024, 2301072. doi: 10.1002/smtd.202301072
- Epshtein, M.; Gounis, M.J.; Bogdanov, A.A. X-ray Attenuating Vesicles with Neutrophil Extracellular Trap (NET) Specificity: Synthesis and Testing in a Model System. ACS Omega, 2024, 9(27), 29391-29400. doi: 10.1021/acsomega.4c01525
- Xing, W.; Li, D.; Wang, W.; Liu, J.-J.G.; Chen, C. Conformational dynamics of CasX (Cas12e) in mediating DNA cleavage revealed by single-molecule FRET. Nucleic Acids Research, 2024. doi: 10.1093/nar/gkae604
Cy® is a trademark of Cytiva.
Short link - lumiprobe.com/sh/p/d
The count of items is incorrect.
























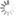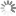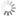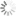
 $
$ 
